What Is The Difference Between Makeup And Structure Of A Molecule
Chapter 7. Chemical Bonding and Molecular Geometry
vii.6 Molecular Structure and Polarity
Learning Objectives
By the end of this section, you will be able to:
- Predict the structures of pocket-sized molecules using valence shell electron pair repulsion (VSEPR) theory
- Explain the concepts of polar covalent bonds and molecular polarity
- Assess the polarity of a molecule based on its bonding and structure
Thus far, we have used 2-dimensional Lewis structures to correspond molecules. Withal, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space (Figure 1). A bond angle is the angle between whatever two bonds that include a common cantlet, usually measured in degrees. A bond distance (or bond length) is the distance between the nuclei of two bonded atoms along the directly line joining the nuclei. Bail distances are measured in Ångstroms (one Å = 10–10 chiliad) or picometers (ane pm = 10–12 m, 100 pm = 1 Å).
VSEPR Theory
Valence shell electron-pair repulsion theory (VSEPR theory) enables usa to predict the molecular structure, including approximate bail angles around a primal atom, of a molecule from an exam of the number of bonds and alone electron pairs in its Lewis structure. The VSEPR model assumes that electron pairs in the valence shell of a central atom volition prefer an organization that minimizes repulsions between these electron pairs by maximizing the distance between them. The electrons in the valence shell of a central atom form either bonding pairs of electrons, located primarily between bonded atoms, or lone pairs. The electrostatic repulsion of these electrons is reduced when the various regions of high electron density assume positions equally far from each other as possible.
VSEPR theory predicts the organisation of electron pairs effectually each central cantlet and, usually, the right arrangement of atoms in a molecule. Nosotros should empathise, however, that the theory but considers electron-pair repulsions. Other interactions, such as nuclear-nuclear repulsions and nuclear-electron attractions, are besides involved in the final arrangement that atoms prefer in a detail molecular structure.
As a simple example of VSEPR theory, let usa predict the construction of a gaseous BeFii molecule. The Lewis structure of BeFtwo (Effigy 2) shows only 2 electron pairs effectually the central glucinium atom. With two bonds and no lone pairs of electrons on the central atom, the bonds are as far autonomously as possible, and the electrostatic repulsion betwixt these regions of loftier electron density is reduced to a minimum when they are on opposite sides of the primal atom. The bond angle is 180° (Effigy 2).
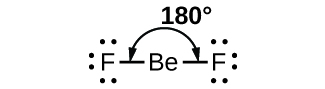
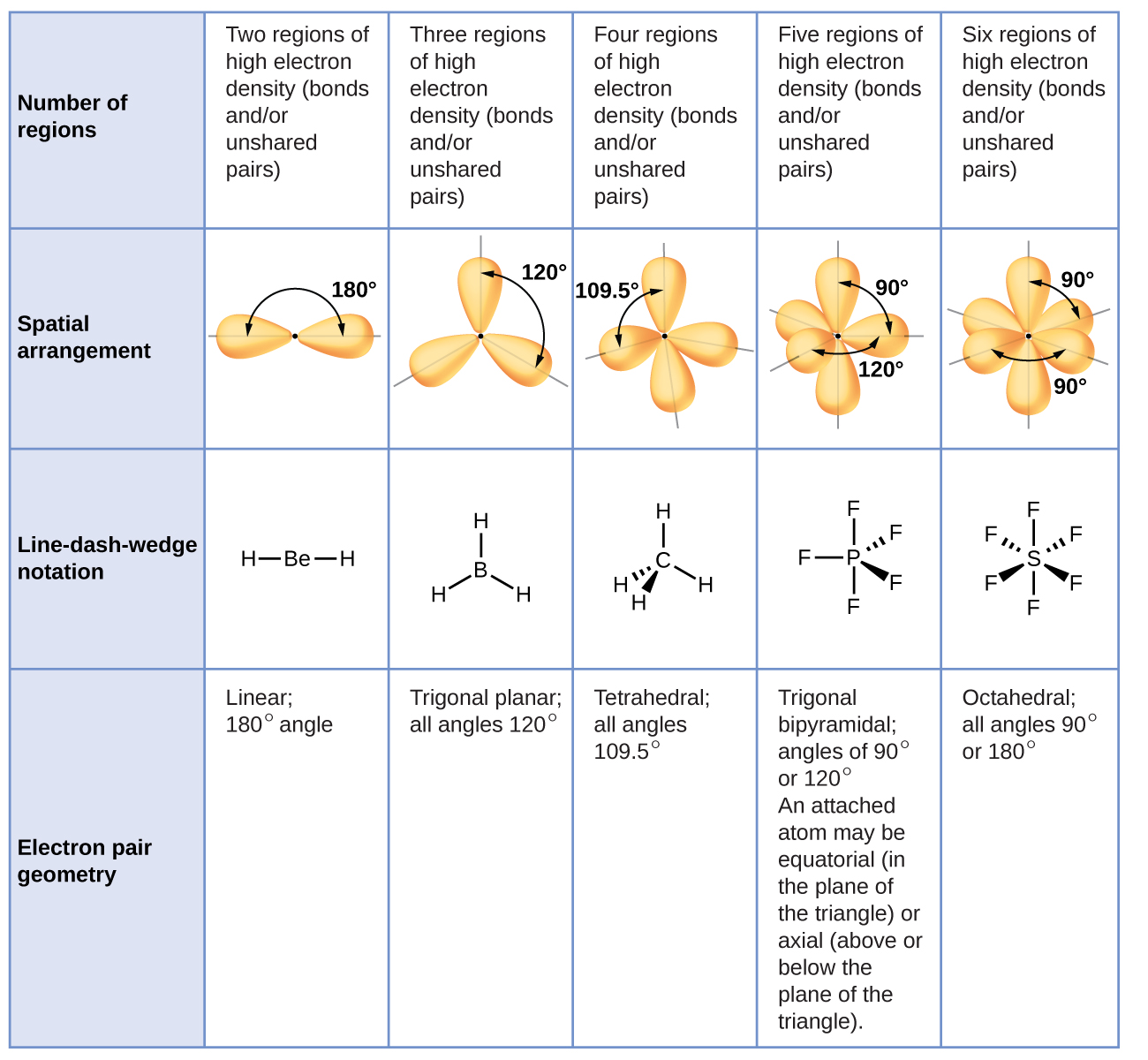
Figure iii illustrates this and other electron-pair geometries that minimize the repulsions amid regions of high electron density (bonds and/or lone pairs). Two regions of electron density around a central atom in a molecule form a linear geometry; iii regions grade a trigonal planar geometry; four regions form a tetrahedral geometry; 5 regions form a trigonal bipyramidal geometry; and half dozen regions form an octahedral geometry.
Electron-pair Geometry versus Molecular Structure
It is important to note that electron-pair geometry around a central cantlet is not the aforementioned thing as its molecular structure. The electron-pair geometries shown in Figure 3 describe all regions where electrons are located, bonds equally well as lone pairs. Molecular structure describes the location of the atoms, not the electrons.
We differentiate between these two situations by naming the geometry that includes all electron pairs the electron-pair geometry. The structure that includes only the placement of the atoms in the molecule is called the molecular structure. The electron-pair geometries volition be the same every bit the molecular structures when at that place are no lonely electron pairs around the central atom, but they will exist unlike when there are lonely pairs present on the central atom.
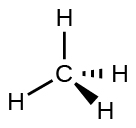
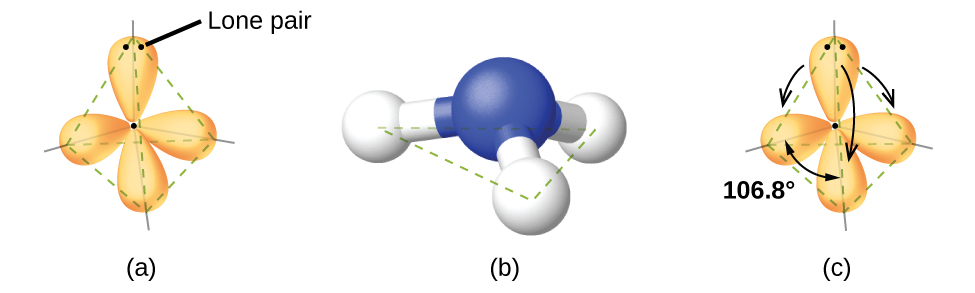
For case, the methane molecule, CHiv, which is the major component of natural gas, has iv bonding pairs of electrons effectually the central carbon cantlet; the electron-pair geometry is tetrahedral, every bit is the molecular structure (Effigy 4). On the other hand, the ammonia molecule, NH3, too has four electron pairs associated with the nitrogen atom, and thus has a tetrahedral electron-pair geometry. One of these regions, however, is a lone pair, which is not included in the molecular structure, and this lone pair influences the shape of the molecule (Effigy 5).
As seen in Effigy v, small distortions from the ideal angles in Figure 3 can result from differences in repulsion between various regions of electron density. VSEPR theory predicts these distortions past establishing an social club of repulsions and an order of the amount of space occupied by different kinds of electron pairs. The social club of electron-pair repulsions from greatest to least repulsion is:
[latex]\text{lone pair-lone pair} > \text{solitary pair-bonding pair} > \text{bonding pair-bonding pair}[/latex]
This order of repulsions determines the corporeality of space occupied by different regions of electrons. A solitary pair of electrons occupies a larger region of infinite than the electrons in a triple bail; in turn, electrons in a triple bail occupy more space than those in a double bond, and then on. The order of sizes from largest to smallest is:
[latex]\text{lone pair} > \text{triple bond} > \text{double bail} > \text{single bail}[/latex]
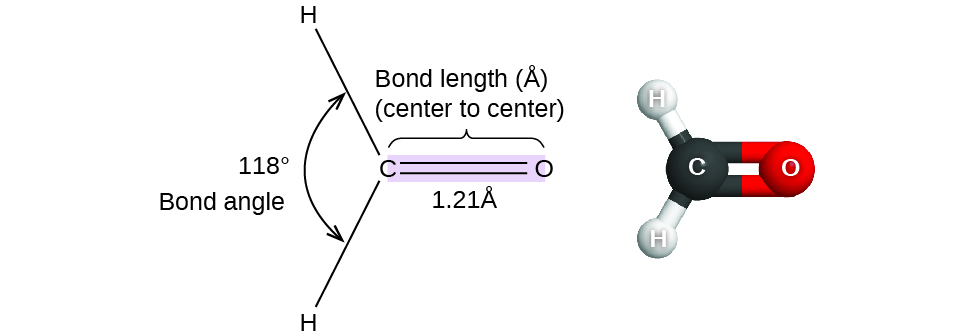
Consider formaldehyde, HtwoCO, which is used as a preservative for biological and anatomical specimens (Figure 1). This molecule has regions of high electron density that consist of 2 single bonds and 1 double bond. The basic geometry is trigonal planar with 120° bond angles, but nosotros see that the double bond causes slightly larger angles (121°), and the angle between the single bonds is slightly smaller (118°).
In the ammonia molecule, the three hydrogen atoms attached to the primal nitrogen are non bundled in a flat, trigonal planar molecular structure, but rather in a three-dimensional trigonal pyramid (Figure 5) with the nitrogen atom at the apex and the three hydrogen atoms forming the base. The ideal bond angles in a trigonal pyramid are based on the tetrahedral electron pair geometry. Over again, there are slight deviations from the ideal considering lone pairs occupy larger regions of space than do bonding electrons. The H–Due north–H bond angles in NH3 are slightly smaller than the 109.v° angle in a regular tetrahedron (Figure iii) because the lone pair-bonding pair repulsion is greater than the bonding pair-bonding pair repulsion (Effigy 5). Figure 6 illustrates the ideal molecular structures, which are predicted based on the electron-pair geometries for various combinations of lone pairs and bonding pairs.
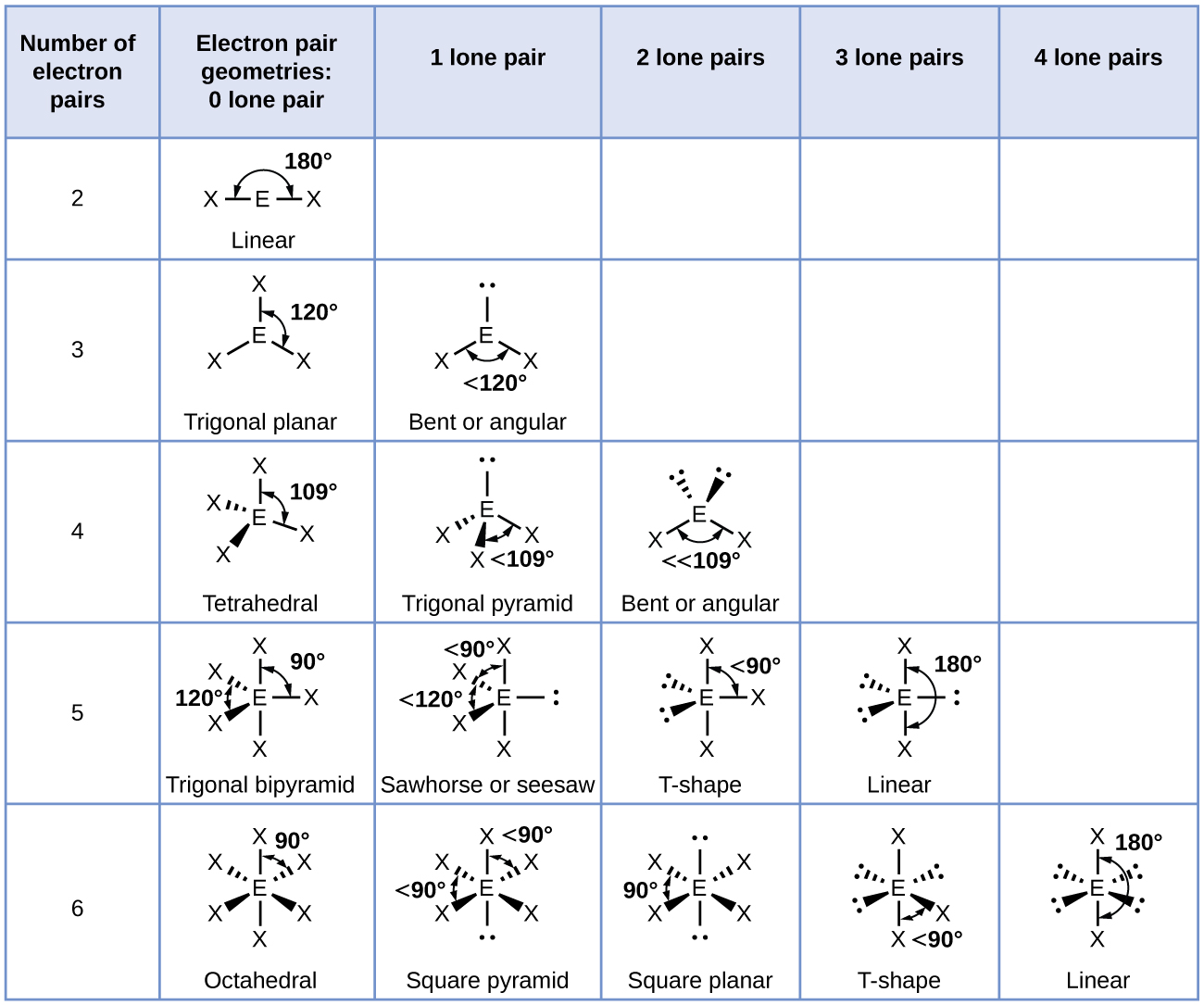
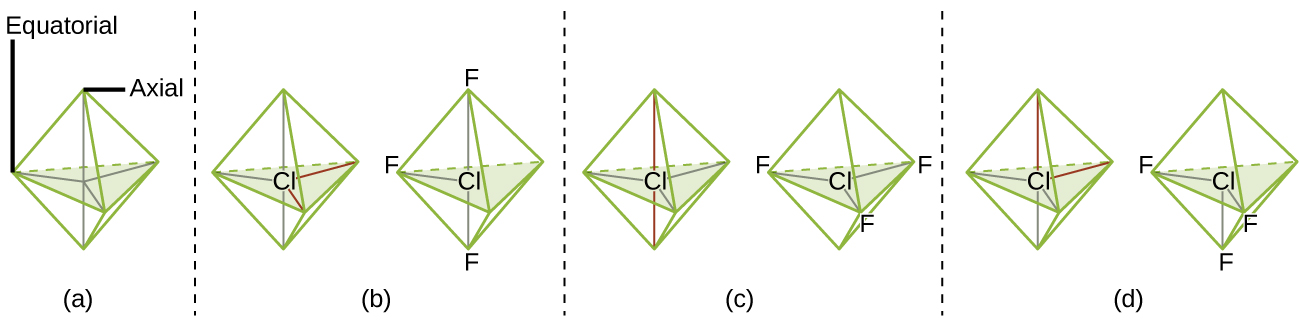
Co-ordinate to VSEPR theory, the terminal atom locations (Xs in Figure half-dozen) are equivalent within the linear, trigonal planar, and tetrahedral electron-pair geometries (the showtime three rows of the table). It does not matter which X is replaced with a solitary pair considering the molecules can exist rotated to convert positions. For trigonal bipyramidal electron-pair geometries, withal, in that location are two distinct X positions, as shown in Effigy 7: an axial position (if we hold a model of a trigonal bipyramid by the two axial positions, nosotros have an axis around which nosotros can rotate the model) and an equatorial position (three positions course an equator around the centre of the molecule). As shown in Figure 6, the axial position is surrounded past bond angles of 90°, whereas the equatorial position has more than space bachelor because of the 120° bond angles. In a trigonal bipyramidal electron-pair geometry, alone pairs always occupy equatorial positions because these more spacious positions can more hands accommodate the larger lone pairs.
Theoretically, we can come up with 3 possible arrangements for the three bonds and two lone pairs for the ClF3 molecule (Figure 7). The stable construction is the i that puts the lone pairs in equatorial locations, giving a T-shaped molecular structure.
When a fundamental atom has ii solitary electron pairs and four bonding regions, we accept an octahedral electron-pair geometry. The two alone pairs are on contrary sides of the octahedron (180° autonomously), giving a square planar molecular construction that minimizes lonely pair-lone pair repulsions (Figure 6).
Predicting Electron Pair Geometry and Molecular Structure
The following process uses VSEPR theory to decide the electron pair geometries and the molecular structures:
- Write the Lewis structure of the molecule or polyatomic ion.
- Count the number of regions of electron density (lonely pairs and bonds) effectually the fundamental atom. A single, double, or triple bond counts as one region of electron density.
- Place the electron-pair geometry based on the number of regions of electron density: linear, trigonal planar, tetrahedral, trigonal bipyramidal, or octahedral (Effigy six, first column).
- Use the number of lone pairs to determine the molecular construction (Figure half dozen). If more than ane organisation of lone pairs and chemic bonds is possible, cull the one that volition minimize repulsions, remembering that lone pairs occupy more than space than multiple bonds, which occupy more infinite than single bonds. In trigonal bipyramidal arrangements, repulsion is minimized when every solitary pair is in an equatorial position. In an octahedral system with two lone pairs, repulsion is minimized when the lone pairs are on opposite sides of the cardinal cantlet.
The following examples illustrate the employ of VSEPR theory to predict the molecular structure of molecules or ions that have no lone pairs of electrons. In this case, the molecular structure is identical to the electron pair geometry.
Example 1
Predicting Electron-pair Geometry and Molecular Structure: CO2 and BCl3
Predict the electron-pair geometry and molecular structure for each of the post-obit:
(a) carbon dioxide, COtwo, a molecule produced by the combustion of fossil fuels
(b) boron trichloride, BClthree, an important industrial chemic
Solution
(a) Nosotros write the Lewis structure of COtwo equally:
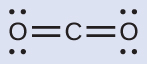
This shows us two regions of high electron density around the carbon atom—each double bond counts as one region, and at that place are no lone pairs on the carbon cantlet. Using VSEPR theory, we predict that the two regions of electron density arrange themselves on reverse sides of the central atom with a bond bending of 180°. The electron-pair geometry and molecular structure are identical, and COtwo molecules are linear.
(b) We write the Lewis structure of BCliii as:
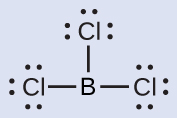
Thus nosotros encounter that BClthree contains 3 bonds, and there are no lone pairs of electrons on boron. The arrangement of three regions of high electron density gives a trigonal planar electron-pair geometry. The B–Cl bonds lie in a plane with 120° angles between them. BCl3 also has a trigonal planar molecular structure (Figure 8).
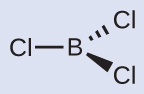
The electron-pair geometry and molecular structure of BCl3 are both trigonal planar. Note that the VSEPR geometry indicates the correct bond angles (120°), different the Lewis construction shown to a higher place.
Bank check Your Learning
Carbonate, COiii ii−, is a common polyatomic ion found in various materials from eggshells to antacids. What are the electron-pair geometry and molecular structure of this polyatomic ion?
Answer:
The electron-pair geometry is trigonal planar and the molecular construction is trigonal planar. Due to resonance, all three C–O bonds are identical. Whether they are single, double, or an boilerplate of the two, each bond counts as one region of electron density.
Example two
Predicting Electron-pair Geometry and Molecular Structure: Ammonium
Two of the top l chemicals produced in the The states, ammonium nitrate and ammonium sulfate, both used equally fertilizers, contain the ammonium ion. Predict the electron-pair geometry and molecular structure of the NHiv + cation.
Solution
We write the Lewis structure of NH4 + as:
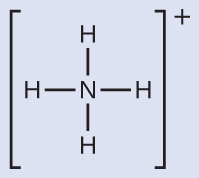
We can see that NH4 + contains four bonds from the nitrogen cantlet to hydrogen atoms and no lone pairs. We expect the iv regions of high electron density to adapt themselves and so that they point to the corners of a tetrahedron with the key nitrogen atom in the heart (Effigy 6). Therefore, the electron pair geometry of NHiv + is tetrahedral, and the molecular structure is besides tetrahedral (Figure 9).
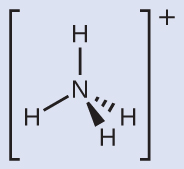
Cheque Your Learning
Place a molecule with trigonal bipyramidal molecular structure.
Answer:
Any molecule with five electron pairs around the central atoms including no lonely pairs will be trigonal bipyramidal. PF5 is a common case.
The next several examples illustrate the event of lone pairs of electrons on molecular structure.
Example 3
Predicting Electron-pair Geometry and Molecular Structure: Lone Pairs on the Central Atom
Predict the electron-pair geometry and molecular structure of a water molecule.
Solution
The Lewis structure of H2O indicates that in that location are four regions of high electron density around the oxygen atom: two lone pairs and ii chemical bonds:
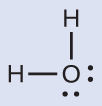
We predict that these four regions are bundled in a tetrahedral fashion (Figure 10), as indicated in Figure half-dozen. Thus, the electron-pair geometry is tetrahedral and the molecular structure is bent with an angle slightly less than 109.5°. In fact, the bail angle is 104.five°.
Check Your Learning
The hydronium ion, H3O+, forms when acids are dissolved in water. Predict the electron-pair geometry and molecular structure of this cation.
Answer:
electron pair geometry: tetrahedral; molecular construction: trigonal pyramidal
Example iv
Predicting Electron-pair Geometry and Molecular Structure: SF4
Sulfur tetrafluoride, SF4, is extremely valuable for the preparation of fluorine-containing compounds used as herbicides (i.e., SFfour is used as a fluorinating agent). Predict the electron-pair geometry and molecular construction of a SF4 molecule.
Solution
The Lewis structure of SF4 indicates five regions of electron density effectually the sulfur cantlet: one alone pair and 4 bonding pairs:
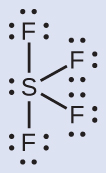
We expect these 5 regions to adopt a trigonal bipyramidal electron-pair geometry. To minimize alone pair repulsions, the lone pair occupies ane of the equatorial positions. The molecular structure (Effigy xi) is that of a seesaw (Figure half dozen).

Check Your Learning
Predict the electron pair geometry and molecular structure for molecules of XeF2.
Answer:
The electron-pair geometry is trigonal bipyramidal. The molecular construction is linear.
Instance v
Predicting Electron-pair Geometry and Molecular Structure: XeF4
Of all the noble gases, xenon is the most reactive, ofttimes reacting with elements such as oxygen and fluorine. Predict the electron-pair geometry and molecular structure of the XeF4 molecule.
Solution
The Lewis structure of XeFiv indicates six regions of high electron density effectually the xenon atom: two lone pairs and iv bonds:
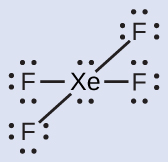
These vi regions adopt an octahedral arrangement (Effigy 6), which is the electron-pair geometry. To minimize repulsions, the solitary pairs should be on opposite sides of the key atom (Figure 12). The five atoms are all in the aforementioned airplane and have a square planar molecular structure.

Check Your Learning
In a sure molecule, the central atom has iii lone pairs and 2 bonds. What will the electron pair geometry and molecular structure be?
Answer:
electron pair geometry: trigonal bipyramidal; molecular structure: linear
Molecular Structure for Multicenter Molecules
When a molecule or polyatomic ion has only one central atom, the molecular construction completely describes the shape of the molecule. Larger molecules practise non have a single central atom, but are connected by a chain of interior atoms that each possess a "local" geometry. The fashion these local structures are oriented with respect to each other also influences the molecular shape, merely such considerations are largely beyond the scope of this introductory give-and-take. For our purposes, nosotros volition only focus on determining the local structures.
Example half dozen
Predicting Structure in Multicenter Molecules
The Lewis structure for the simplest amino acrid, glycine, H2NCH2CO2H, is shown hither. Predict the local geometry for the nitrogen atom, the two carbon atoms, and the oxygen cantlet with a hydrogen atom attached:
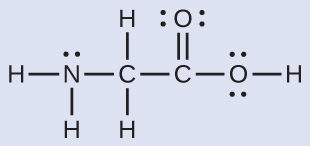
Solution
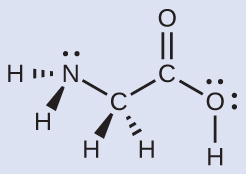
Consider each central atom independently. The electron-pair geometries:
- nitrogen––iv regions of electron density; tetrahedral
- carbon (CHii)––four regions of electron density; tetrahedral
- carbon (CO2)—three regions of electron density; trigonal planar
- oxygen (OH)—iv regions of electron density; tetrahedral
The local structures:
- nitrogen––three bonds, i lonely pair; trigonal pyramidal
- carbon (CH2)—four bonds, no lone pairs; tetrahedral
- carbon (CO2)—three bonds (double bond counts as 1 bond), no alone pairs; trigonal planar
- oxygen (OH)—2 bonds, two lone pairs; aptitude (109°)
Bank check Your Learning
Another amino acrid is alanine, which has the Lewis structure shown here. Predict the electron-pair geometry and local structure of the nitrogen cantlet, the three carbon atoms, and the oxygen cantlet with hydrogen attached:
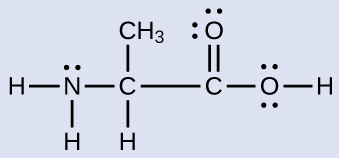
Answer:
electron-pair geometries: nitrogen––tetrahedral; carbon (CH)—tetrahedral; carbon (CH3)—tetrahedral; carbon (COtwo)—trigonal planar; oxygen (OH)—tetrahedral; local structures: nitrogen—trigonal pyramidal; carbon (CH)—tetrahedral; carbon (CH3)—tetrahedral; carbon (COtwo)—trigonal planar; oxygen (OH)—aptitude (109°)

The molecular shape simulator lets yous build various molecules and practice naming their electron-pair geometries and molecular structures.
Example seven
Molecular Simulation
Using molecular shape simulator allows usa to control whether bond angles and/or solitary pairs are displayed past checking or unchecking the boxes under "Options" on the correct. We can also apply the "Name" checkboxes at lesser-left to brandish or hide the electron pair geometry (chosen "electron geometry" in the simulator) and/or molecular structure (called "molecular shape" in the simulator).
Build the molecule HCN in the simulator based on the following Lewis construction:
[latex]\text{H} - \text{C} \equiv \text{N}[/latex]
Click on each bail type or lone pair at right to add that group to the fundamental atom. Once you lot have the complete molecule, rotate it to examine the predicted molecular construction. What molecular structure is this?
Solution
The molecular structure is linear.
Cheque Your Learning
Build a more complex molecule in the simulator. Identify the electron-group geometry, molecular construction, and bail angles. And so try to notice a chemical formula that would match the structure y'all accept drawn.
Respond:
Answers volition vary. For example, an atom with 4 single bonds, a double bond, and a lone pair has an octahedral electron-group geometry and a square pyramidal molecular construction. XeOFfour is a molecule that adopts this structure.
Molecular Polarity and Dipole Moment
As discussed previously, polar covalent bonds connect two atoms with differing electronegativities, leaving one atom with a partial positive charge (δ+) and the other atom with a partial negative charge (δ–), as the electrons are pulled toward the more electronegative atom. This separation of charge gives rise to a bail dipole moment. The magnitude of a bond dipole moment is represented by the Greek letter mu (µ) and is given by the formula shown hither, where Q is the magnitude of the fractional charges (determined by the electronegativity difference) and r is the altitude betwixt the charges:
[latex]\mu = \text{Qr}[/latex]
This bond moment can exist represented as a vector, a quantity having both management and magnitude (Figure 13). Dipole vectors are shown every bit arrows pointing along the bail from the less electronegative atom toward the more electronegative cantlet. A pocket-sized plus sign is drawn on the less electronegative stop to betoken the partially positive end of the bond. The length of the arrow is proportional to the magnitude of the electronegativity difference betwixt the two atoms.
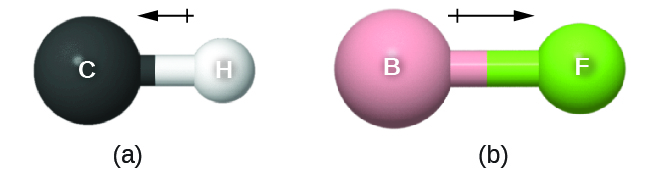
A whole molecule may likewise accept a separation of accuse, depending on its molecular structure and the polarity of each of its bonds. If such a charge separation exists, the molecule is said to be a polar molecule (or dipole); otherwise the molecule is said to be nonpolar. The dipole moment measures the extent of net accuse separation in the molecule every bit a whole. We determine the dipole moment by adding the bond moments in three-dimensional space, taking into account the molecular structure.
For diatomic molecules, there is just ane bond, so its bond dipole moment determines the molecular polarity. Homonuclear diatomic molecules such as Br2 and N2 have no difference in electronegativity, and so their dipole moment is zero. For heteronuclear molecules such as CO, in that location is a small dipole moment. For HF, in that location is a larger dipole moment because at that place is a larger deviation in electronegativity.
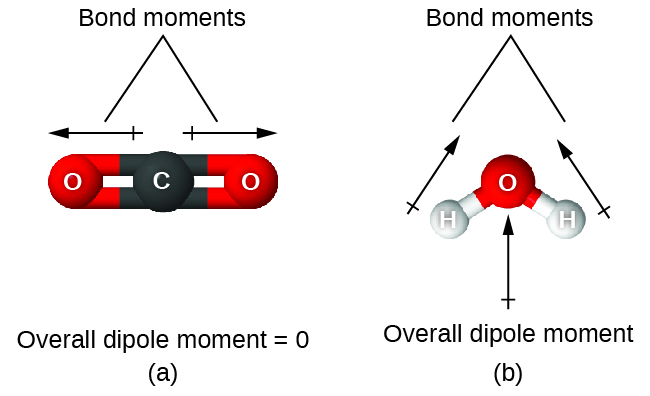
When a molecule contains more than 1 bond, the geometry must be taken into business relationship. If the bonds in a molecule are arranged such that their bond moments cancel (vector sum equals zero), then the molecule is nonpolar. This is the state of affairs in COii (Figure 14). Each of the bonds is polar, only the molecule as a whole is nonpolar. From the Lewis structure, and using VSEPR theory, nosotros determine that the COii molecule is linear with polar C=O bonds on contrary sides of the carbon atom. The bond moments cancel considering they are pointed in opposite directions. In the example of the h2o molecule (Effigy 14), the Lewis structure over again shows that there are ii bonds to a central cantlet, and the electronegativity departure again shows that each of these bonds has a nonzero bond moment. In this case, however, the molecular structure is bent considering of the lone pairs on O, and the two bond moments do not cancel. Therefore, water does take a net dipole moment and is a polar molecule (dipole).
The OCS molecule has a construction similar to COii, merely a sulfur atom has replaced one of the oxygen atoms. To determine if this molecule is polar, we describe the molecular construction. VSEPR theory predicts a linear molecule:
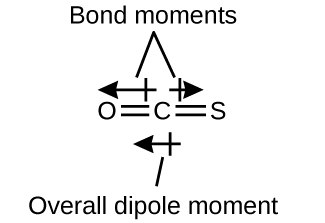
The C-O bond is considerably polar. Although C and S have very similar electronegativity values, S is slightly more than electronegative than C, and and then the C-S bond is just slightly polar. Because oxygen is more electronegative than sulfur, the oxygen finish of the molecule is the negative end.
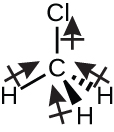
Chloromethane, CH3Cl, is another example of a polar molecule. Although the polar C–Cl and C–H bonds are arranged in a tetrahedral geometry, the C–Cl bonds have a larger bond moment than the C–H bond, and the bond moments do non completely cancel each other. All of the dipoles have a down component in the orientation shown, since carbon is more electronegative than hydrogen and less electronegative than chlorine:
When we examine the highly symmetrical molecules BF3 (trigonal planar), CHiv (tetrahedral), PF5 (trigonal bipyramidal), and SFsix (octahedral), in which all the polar bonds are identical, the molecules are nonpolar. The bonds in these molecules are arranged such that their dipoles abolish. However, only because a molecule contains identical bonds does not hateful that the dipoles will always abolish. Many molecules that have identical bonds and lone pairs on the cardinal atoms have bail dipoles that exercise not cancel. Examples include HiiS and NH3. A hydrogen atom is at the positive end and a nitrogen or sulfur atom is at the negative finish of the polar bonds in these molecules:
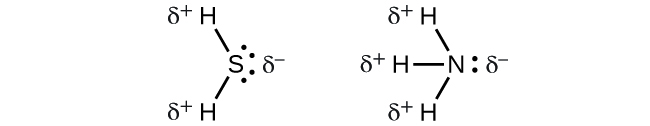
To summarize, to exist polar, a molecule must:
- Incorporate at least one polar covalent bond.
- Take a molecular structure such that the sum of the vectors of each bond dipole moment does not cancel.
Backdrop of Polar Molecules
Polar molecules tend to align when placed in an electric field with the positive finish of the molecule oriented toward the negative plate and the negative end toward the positive plate (Figure xv). We can use an electrically charged object to concenter polar molecules, but nonpolar molecules are not attracted. Also, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances.
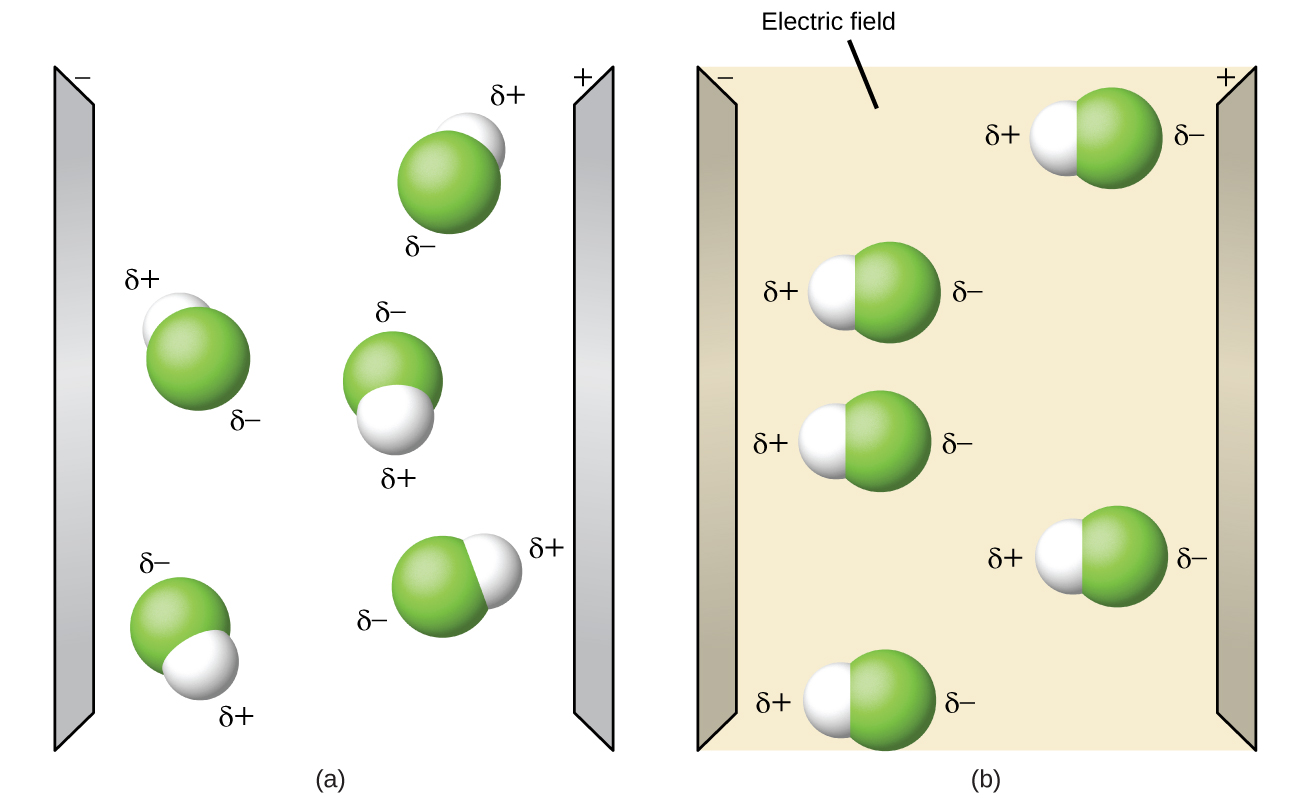

The molecule polarity simulation provides many ways to explore dipole moments of bonds and molecules.
Instance 8
Polarity Simulations
Open the molecule polarity simulation and select the "Three Atoms" tab at the top. This should display a molecule ABC with iii electronegativity adjustors. You tin display or hide the bail moments, molecular dipoles, and partial charges at the right. Turning on the Electric Field will show whether the molecule moves when exposed to a field, like to Effigy xv.
Employ the electronegativity controls to make up one's mind how the molecular dipole will look for the starting bent molecule if:
(a) A and C are very electronegative and B is in the centre of the range.
(b) A is very electronegative, and B and C are not.
Solution
(a) Molecular dipole moment points immediately between A and C.
(b) Molecular dipole moment points along the A–B bond, toward A.
Check Your Learning
Decide the partial charges that volition give the largest possible bond dipoles.
Respond:
The largest bail moments will occur with the largest fractional charges. The two solutions above stand for how unevenly the electrons are shared in the bond. The bail moments volition be maximized when the electronegativity difference is greatest. The controls for A and C should be prepare to one extreme, and B should be set to the opposite farthermost. Although the magnitude of the bond moment will non change based on whether B is the most electronegative or the least, the direction of the bond moment will.
Key Concepts and Summary
VSEPR theory predicts the three-dimensional arrangement of atoms in a molecule. Information technology states that valence electrons will presume an electron-pair geometry that minimizes repulsions between areas of loftier electron density (bonds and/or lone pairs). Molecular structure, which refers only to the placement of atoms in a molecule and not the electrons, is equivalent to electron-pair geometry only when there are no lone electron pairs around the key atom. A dipole moment measures a separation of charge. For one bond, the bond dipole moment is determined by the difference in electronegativity between the ii atoms. For a molecule, the overall dipole moment is determined by both the individual bail moments and how these dipoles are bundled in the molecular structure. Polar molecules (those with an observable dipole moment) interact with electric fields, whereas nonpolar molecules do not.
Chemistry End of Chapter Exercises
- Explain why the HOH molecule is bent, whereas the HBeH molecule is linear.
- What characteristic of a Lewis structure tin be used to tell if a molecule's (or ion's) electron-pair geometry and molecular structure will exist identical?
- Explain the difference betwixt electron-pair geometry and molecular structure.
- Why is the H–N–H bending in NH3 smaller than the H–C–H bail angle in CH4? Why is the H–N–H bending in NHiv + identical to the H–C–H bail angle in CH4?
- Explain how a molecule that contains polar bonds can be nonpolar.
- As a general rule, MXn molecules (where M represents a central atom and X represents final atoms; north = 2 – v) are polar if there is one or more lonely pairs of electrons on M. NH3 (Grand = N, X = H, northward = 3) is an example. In that location are two molecular structures with lone pairs that are exceptions to this dominion. What are they?
- Predict the electron pair geometry and the molecular construction of each of the post-obit molecules or ions:
(a) SFhalf dozen
(b) PClv
(c) BeH2
(d) CH3 +
- Identify the electron pair geometry and the molecular structure of each of the following molecules or ions:
(a) IF6 +
(b) CFfour
(c) BFthree
(d) SiF5 −
(e) BeCl2
- What are the electron-pair geometry and the molecular construction of each of the following molecules or ions?
(a) ClF5
(b) ClOii −
(c) TeCl4 two−
(d) PCl3
(e) SeF4
(f) PH2 −
- Predict the electron pair geometry and the molecular structure of each of the following ions:
(a) H3O+
(b) PClfour −
(c) SnCl3 −
(d) BrCl4 −
(e) ICl3
(f) XeFfour
(thousand) SFtwo
- Identify the electron pair geometry and the molecular structure of each of the following molecules:
(a) ClNO (N is the cardinal atom)
(b) CS2
(c) Cl2CO (C is the central atom)
(d) Cl2And then (South is the primal atom)
(e) SOtwoFtwo (S is the central cantlet)
(f) XeO2F2 (Xe is the central cantlet)
(g) ClOF2+ (Cl is the central cantlet)
- Predict the electron pair geometry and the molecular construction of each of the following:
(a) IOF5 (I is the primal atom)
(b) POCliii (P is the fundamental atom)
(c) Cl2SeO (Se is the central cantlet)
(d) ClSO+ (South is the central cantlet)
(e) FiiSO (Due south is the central cantlet)
(f) NOtwo −
(g) SiO4 four−
- Which of the following molecules and ions contain polar bonds? Which of these molecules and ions take dipole moments?
(a) ClF5
(b) ClOii −
(c) TeCl4 2−
(d) PCl3
(due east) SeFiv
(f) PHtwo −
(g) XeFii
- Which of these molecules and ions incorporate polar bonds? Which of these molecules and ions have dipole moments?
(a) H3O+
(b) PCl4 −
(c) SnCliii −
(d) BrCl4 −
(due east) ICl3
(f) XeF4
(yard) SFii
- Which of the following molecules have dipole moments?
(a) CS2
(b) SeS2
(c) CCltwoF2
(d) PClthree (P is the primal cantlet)
(e) ClNO (N is the central atom)
- Identify the molecules with a dipole moment:
(a) SFiv
(b) CFfour
(c) Cl2CCBr2
(d) CH3Cl
(eastward) H2CO
- The molecule XF3 has a dipole moment. Is X boron or phosphorus?
- The molecule XClii has a dipole moment. Is X beryllium or sulfur?
- Is the Cl2BBCl2 molecule polar or nonpolar?
- There are three possible structures for PCl2F3 with phosphorus as the cardinal atom. Describe them and discuss how measurements of dipole moments could help distinguish among them.
- Describe the molecular structure around the indicated atom or atoms:
(a) the sulfur atom in sulfuric acid, HtwoSO4 [(HO)twoAnd so2]
(b) the chlorine atom in chloric acid, HClOiii [HOClOtwo]
(c) the oxygen atom in hydrogen peroxide, HOOH
(d) the nitrogen cantlet in nitric acid, HNO3 [HONO2]
(east) the oxygen atom in the OH grouping in nitric acrid, HNO3 [HONO2]
(f) the central oxygen atom in the ozone molecule, Othree
(thousand) each of the carbon atoms in propyne, CH3CCH
(h) the carbon cantlet in Freon, CCltwoFtwo
(i) each of the carbon atoms in allene, H2CCCH2
- Describe the Lewis structures and predict the shape of each chemical compound or ion:
(a) CO2
(b) NO2 −
(c) SOiii
(d) Then3 ii−
- A molecule with the formula AB2, in which A and B stand for unlike atoms, could have one of 3 different shapes. Sketch and name the three unlike shapes that this molecule might have. Give an example of a molecule or ion for each shape.
- A molecule with the formula AB3, in which A and B represent different atoms, could have one of iii different shapes. Sketch and name the iii different shapes that this molecule might have. Give an instance of a molecule or ion that has each shape.
- Draw the Lewis electron dot structures for these molecules, including resonance structures where appropriate:
(a) CS3 2−
(b) CStwo
(c) CS
(d) predict the molecular shapes for CSiii two− and CSii and explain how you lot arrived at your predictions
- What is the molecular structure of the stable grade of FNO2? (Northward is the central atom.)
- A chemical compound with a molar mass of about 42 g/mol contains 85.7% carbon and 14.three% hydrogen. What is its molecular structure?
- Utilise the simulation to perform the post-obit exercises for a ii-atom molecule:
(a) Conform the electronegativity value so the bond dipole is pointing toward B. And then determine what the electronegativity values must be to switch the dipole so that information technology points toward A.
(b) With a partial positive charge on A, turn on the electric field and describe what happens.
(c) With a small partial negative charge on A, turn on the electric field and depict what happens.
(d) Reset all, and so with a big fractional negative charge on A, turn on the electric field and describe what happens.
- Use the simulation to perform the following exercises for a real molecule. You may need to rotate the molecules in iii dimensions to see certain dipoles.
(a) Sketch the bond dipoles and molecular dipole (if any) for Oiii. Explain your observations.
(b) Look at the bail dipoles for NHiii. Use these dipoles to predict whether Northward or H is more electronegative.
(c) Predict whether at that place should be a molecular dipole for NH3 and, if and so, in which direction it volition betoken. Cheque the molecular dipole box to test your hypothesis.
- Utilise the Molecule Shape simulator to build a molecule. Starting with the primal atom, click on the double bond to add one double bond. So add ane unmarried bail and ane lone pair. Rotate the molecule to observe the complete geometry. Name the electron group geometry and molecular construction and predict the bond angle. So click the check boxes at the bottom and right of the simulator to check your answers.
- Apply the Molecule Shape simulator to explore real molecules. On the Real Molecules tab, select H2O. Switch between the "existent" and "model" modes. Explain the difference observed.
- Use the Molecule Shape simulator to explore existent molecules. On the Real Molecules tab, select "model" mode and Due south2O. What is the model bond angle? Explicate whether the "real" bond angle should be larger or smaller than the ideal model angle.
Glossary
- axial position
- location in a trigonal bipyramidal geometry in which there is another atom at a 180° bending and the equatorial positions are at a 90° angle
- bail angle
- angle between any ii covalent bonds that share a mutual atom
- bail distance
- (also, bond length) altitude between the nuclei of ii bonded atoms
- bail dipole moment
- separation of charge in a bond that depends on the difference in electronegativity and the bond distance represented by partial charges or a vector
- dipole moment
- property of a molecule that describes the separation of charge determined by the sum of the individual bond moments based on the molecular structure
- electron-pair geometry
- arrangement effectually a cardinal atom of all regions of electron density (bonds, lone pairs, or unpaired electrons)
- equatorial position
- one of the three positions in a trigonal bipyramidal geometry with 120° angles between them; the centric positions are located at a xc° angle
- linear
- shape in which two outside groups are placed on opposite sides of a cardinal atom
- molecular structure
- structure that includes only the placement of the atoms in the molecule
- octahedral
- shape in which half-dozen outside groups are placed around a central atom such that a 3-dimensional shape is generated with four groups forming a square and the other two forming the apex of two pyramids, i above and one beneath the square plane
- polar molecule
- (likewise, dipole) molecule with an overall dipole moment
- tetrahedral
- shape in which 4 outside groups are placed around a key atom such that a three-dimensional shape is generated with 4 corners and 109.5° angles between each pair and the central atom
- trigonal bipyramidal
- shape in which five exterior groups are placed around a central atom such that three form a flat triangle with 120° angles between each pair and the central cantlet, and the other two form the noon of 2 pyramids, one higher up and one below the triangular plane
- trigonal planar
- shape in which three outside groups are placed in a flat triangle around a cardinal atom with 120° angles betwixt each pair and the central atom
- valence shell electron-pair repulsion theory (VSEPR)
- theory used to predict the bond angles in a molecule based on positioning regions of loftier electron density as far apart as possible to minimize electrostatic repulsion
- vector
- quantity having magnitude and direction
Solutions
Answers to Chemistry Finish of Chapter Exercises
1. The placement of the 2 sets of unpaired electrons in water forces the bonds to assume a tetrahedral arrangement, and the resulting HOH molecule is aptitude. The HBeH molecule (in which Be has only two electrons to bond with the ii electrons from the hydrogens) must have the electron pairs as far from one some other every bit possible and is therefore linear.
3. Space must exist provided for each pair of electrons whether they are in a bond or are present as lone pairs. Electron-pair geometry considers the placement of all electrons. Molecular structure considers just the bonding-pair geometry.
5. As long equally the polar bonds are compensated (for example. two identical atoms are establish direct across the central cantlet from one another), the molecule can exist nonpolar.
7. (a) Both the electron geometry and the molecular structure are octahedral.
(b) Both the electron geometry and the molecular structure are trigonal bipyramid.
(c) Both the electron geometry and the molecular structure are linear.
(d) Both the electron geometry and the molecular structure are trigonal planar.
9. (a) electron-pair geometry: octahedral, molecular structure: square pyramidal; (b) electron-pair geometry: tetrahedral, molecular structure: bent; (c) electron-pair geometry: octahedral, molecular structure: square planar; (d) electron-pair geometry: tetrahedral, molecular construction: trigonal pyramidal; (due east) electron-pair geometry: trigonal bypyramidal, molecular structure: seesaw; (f) electron-pair geometry: tetrahedral, molecular structure: bent (109°)
11. (a) electron-pair geometry: trigonal planar, molecular structure: aptitude (120°); (b) electron-pair geometry: linear, molecular construction: linear; (c) electron-pair geometry: trigonal planar, molecular structure: trigonal planar; (d) electron-pair geometry: tetrahedral, molecular construction: trigonal pyramidal; (e) electron-pair geometry: tetrahedral, molecular structure: tetrahedral; (f) electron-pair geometry: trigonal bipyramidal, molecular construction: seesaw; (1000) electron-pair geometry: tetrahedral, molecular construction: trigonal pyramidal
xiii. All of these molecules and ions contain polar bonds. Only ClF5, ClO2 −, PClthree, SeF4, and PH2 − have dipole moments.
15.SeS2, CCl2F2, PCliii, and ClNO all have dipole moments.
17. P
19. nonpolar
21. (a) tetrahedral; (b) trigonal pyramidal; (c) bent (109°); (d) trigonal planar; (e) bent (109°); (f) bent (109°); (one thousand) CH3CCH tetrahedral, CH3 CCH linear; (h) tetrahedral; (i) H2CCCHii linear; Htwo CCCH2 trigonal planar
23.

25.
(a)
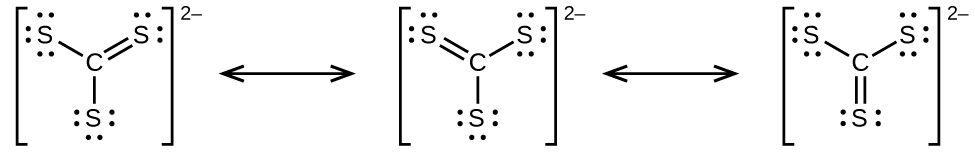 ;
;
(b)
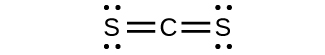 ;
;
(c)
 ;
;
(d) CS3 two− includes iii regions of electron density (all are bonds with no solitary pairs); the shape is trigonal planar; CStwo has only two regions of electron density (all bonds with no lonely pairs); the shape is linear
27. The Lewis structure is made from three units, merely the atoms must be rearranged:
29. The molecular dipole points away from the hydrogen atoms.
31. The structures are very like. In the model mode, each electron grouping occupies the aforementioned corporeality of infinite, then the bail angle is shown as 109.5°. In the "real" mode, the solitary pairs are larger, causing the hydrogens to exist compressed. This leads to the smaller angle of 104.5°.
Source: https://opentextbc.ca/chemistry/chapter/7-6-molecular-structure-and-polarity/
Posted by: pachecoambee1997.blogspot.com

0 Response to "What Is The Difference Between Makeup And Structure Of A Molecule"
Post a Comment